| June 2009 | Print Article |
Comparative Biosciences Phone: 408.738.9260 |
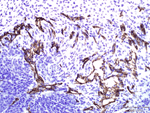
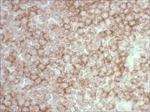
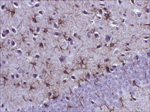
Antibody Cross-Reactivity Studies at Comparative Biosciences Did you know that when submitting an IND for a therapeutic biological molecule, the FDA now requires that all monoclonal antibodies and other biologics undergo tissue cross-reactivity testing? This is necessary to ensure that the antibody does not bind to receptors or other epitopes in addition to the intended target. In addition, non-GLP, or experimental Tissue Cross-Reactivity studies, is a key tool used to identify potential species for GLP toxicology studies for IND submission. Comparative Biosciences, Inc conducts Tissue Cross-Reactivity studies in accordance with the F.D.A.s’ guidance document, "Points to Consider in the Manufacture and Testing of Monoclonal Antibody Products for Human Use," under GLP guidelines. Tissue Cross-Reactivity Studies are comprised of three distinct phases, often preceded by a staining method development phase: Phase I - Antibody Preparation and Characterization Phase II - Preliminary TCR Screen: Intended to identify potential cross-reactivity issues prior to conducting the full screen. Phase III - Comprehensive TCR Screen: Both the test and IgG isotype control antibodies are used at a high and a low concentration to immunolabel 3 individual specimens each of the approximately 33 frozen normal tissue types recommended by the FDA.
Comparative Biosciences, Inc. TCR services include:
Contact Taylor Norwitt, Business Development Manager today at 408-504-8871 to schedule a tour or to discuss your TCR study requirements. Copyright © 2009 Comparative Biosciences, Inc. All rights reserved |